Background: Jaktinib, a novel Janus kinase (JAK) and ACVR1 inhibitor, demonstrated promising clinical benefit with good tolerability, not only efficiently shrinking the spleen and reducing symptom burdens, but also improving anemia, in JAK-inhibitor naïve, ruxolitinib-intolerant or -resistant MF patients. We conducted a phase 2 study (the ZGJAK002 trial) to evaluate the effectiveness and safety of jaktinib at doses of 100 mg BID and 200 mg QD in JAK-inhibitor naïve patients with intermediate or high risk MF. Herein we present long-term data with a median follow-up time of 30.7 months.
Methods: Patients who completed the 24 weeks of study treatment, tolerated the drug well, and had evidence of clinically significant improvement were allowed to enter an extension phase. Dose modifications were permitted according to the platelet count and neutrophil count. Duration of spleen response (from the first spleen volume reduction of ≥35% [SVR35] to an increase of 25% or more from the nadir), overall survival (OS), and leukemia-free survival (LFS) were evaluated in an intent-to-treat analysis using a Cox proportional hazard model that estimated the treatment effect.
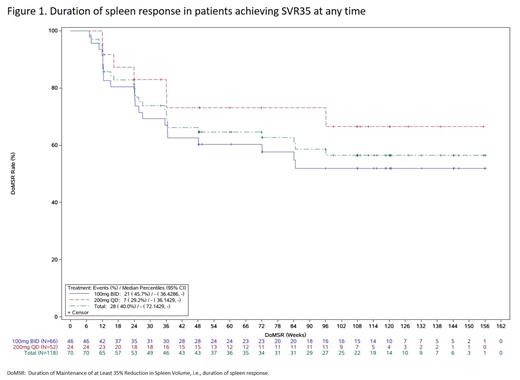
Results: A total of 118 patients were enrolled and treated with either jaktinib 100 mg BID (n=66) or 200 mg QD (n=52). The majority of patients remained on treatment after 48 weeks (100 mg BID group: 74.2%; 200 mg QD group: 65.4%). At the data cutoff of June 30, 2022, treatment was continued in 35 (29.7%) patients (30.3% in BID; 28.8% in QD). The best spleen response, defined as SVR35 at any time, was achieved by 59.3% (95% confidence interval [CI]: 49.9%-68.3%) of patients. The 100 mg BID group demonstrated a greater best spleen response (69.7% [95% CI: 57.1%-80.4%]) compared to the 200 mg QD group (46.2% [95% CI: 32.2%-60.5%]). The median maximum reduction from baseline in spleen volume was 49.7%. The median duration of spleen response was not reached, with 51.9% (95% CI: 36.0%-65.7%) of patients from 100 mg BID and 66.5% (95% CI: 41.4%-82.8%) from 200 mg QD maintaining a spleen response for over 108 weeks (Figure 1). The proportion of patients who achieved an improvement of ≥50% in the TSS (TSS50) was 69.6% (95% CI: 55.9%-81.2%) at week 24 and 74.2% (95% CI: 55.4%-88.1%) at week 108 in the 100 mg BID group. The proportion of patients who achieved TSS50 was 57.5% (95% CI: 40.9%-73.0%) at week 24 and 66.7% (95% CI: 44.7%-84.4%) at week 108 in the 200 mg QD group. As of the data cutoff, 25 (21.2%) deaths were reported (12 [18.2%] of 66 in the 100 mg BID group and 13 [25.0%] of 52 patients in the 200 mg QD group). Median OS was not reached, with 12, 24, and 36-month survival rates of 92.3%, 85.8%, and 78.2% in the 100 mg BID and 92.3%, 78.0%, and 73.6% in 200 mg QD groups. Median LFS was not reached, with 12, 24, and 36-month LFS rates of 94.8%, 90.6%, and 84.1% in the 100 mg BID and 95.5%, 85.7%, and 85.7% in 200 mg QD groups. The 100 mg BID induced more increase from baseline in hemoglobin concentration over 144 weeks with a range of 2.18-10.17 g/L compared to -1.74-1.96 g/L with 200 mg QD. The median (range) duration of jaktinib exposure was 672.0 (14-1262) days in the 100 mg BID group and 588.5 (42-1175) days in the 200 mg QD group. The median relative dose intensity for jaktinib was 97.5% (98.1% in BID; 96.3% in QD). There was no unexpected increased incidence of adverse events (AEs) with longer exposure. The most common grade ≥3 adverse drug reactions were anemia (15.2% vs. 21.2%), thrombocytopenia (15.2% vs. 11.5%), and infectious pneumonia (10.6% vs. 1.9%) in the 100 mg BID and 200 mg QD groups, respectively. Notably, infectious pneumonia (10.6%), thrombocytopenia (4.5%), and peripheral neuropathy (3.0%) were the most common drug-related serious AEs. AEs leading to treatment discontinuation occurred in 14 patients (11.9%), with higher rate in the 100 mg BID group compared to the 200 mg QD group (15.2% vs. 7.7%). AEs resulted in death in seven (5.9%) patients, with similar rates in both groups (6.1% in BID; 5.8% in QD).
Conclusions: With over 30-month follow-up, jaktinib at doses of 100 mg BID and 200 mg QD demonstrated durable high effective rates and improved survival rates without new safety issue. The long-term data further supports the recommendation of 100 mg BID over 200 mg QD administration. It also concluded that jaktinib remains the treatment strategy that offers excellent chances for improving survival in MF patients.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal